costimulation
Costimulation involves ligand-receptor interactions at the surfaces of a responder lymphocyte and an "accessory" cell – APCs for activation of T cells, and helper T cells for activation of B cells.
▼activation B : activation T : anergy : CD28 receptor : CD28RE : CDC42 : costimulatory molecules : first/second signals : helper T cell : IL-2 : MAPK cascade : MHC class II : negative regulators : Rac : regulatory mechanisms : Rel-NFkB : Rho GTPases : TCR engagement : TCR threshold reduction : transcription factors : WASP ▼
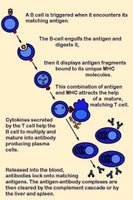 Activation of B cells occurs when a BCR (antibody) encounters and ligates its cognate antigen. Naïve B cells each have one of millions of distinct surface antigen-specific receptors, yet have not encountered their specific, cognate antigen. With a life-span of only a few days, many B cells die without ever encountering their cognate antigen. In most cases, B-cell activation is dependent upon costimulation by an activated helper T cell that has itself been activated by the same antigen. (click images to enlarge)
Activation of B cells occurs when a BCR (antibody) encounters and ligates its cognate antigen. Naïve B cells each have one of millions of distinct surface antigen-specific receptors, yet have not encountered their specific, cognate antigen. With a life-span of only a few days, many B cells die without ever encountering their cognate antigen. In most cases, B-cell activation is dependent upon costimulation by an activated helper T cell that has itself been activated by the same antigen. (click images to enlarge)
Unlike T cells, B cells are coated in immunoglobulin receptors and are able to recognize intact antigen, which they engulf, digest, and subsequently present in complex with surface MHC class II molecules. The MHC-peptide complex binds CD4 + helper T cells (Th), inducing secretion of cytokines that stimulate B cell proliferation and their differentiation into plasma cells, which secrete specific antibodies that bind with the cognate antigen. These antigen-antibody complexes are subsequently cleared by liver and spleen cells and the classical complement cascade.
 Activation of T cells requires (1) TCR engagement, which ensures antigen specificity and MHC restriction of the response. However, synergistic signaling by (2) costimulatory molecules is also necessary to sustain and integrate TCR signaling to stimulate optimal T cell proliferation and differentiation.
Activation of T cells requires (1) TCR engagement, which ensures antigen specificity and MHC restriction of the response. However, synergistic signaling by (2) costimulatory molecules is also necessary to sustain and integrate TCR signaling to stimulate optimal T cell proliferation and differentiation.
Delivery of first signal (TCR engagement) in the absence of costimulation by a second signal(s) results in apoptosis or anergy. Anergic T cells neither produce IL-2 nor proliferate upon restimulation. This requirement of naïve T cell activation for delivery of both antigen-specific and costimulatory signals implies that only professional antigen presenting cells can initiate T cell responses.
Activation-regulatory mechanisms:
● increasing TCR avidity (adhesion molecules)
● enhancing recruitment of tyrosine kinases to the TCR complex coreceptors (CD4 and CD8)
● costimulation involving reciprocal and sequential signals between cells
Negative regulators of costimulation include receptors that bind B7 family members:
● CTLA-4
● PD-1
Molecules involved in costimulation include:
1. Disulfide-linked homodimers that bind to distinct members of the B7 family of surface proteins
---● CD28 ↓
---● ICOS (inducible costimulator) molecules
2. Members of the TNF receptor (TNFR) family
---● CD40, the major B cell costimulatory molecule
---● CD30
---● CD27
---● OX-40
---● 4-1BB
The CD28 receptor is involved in the best characterized costimulatory pathway. CD28 is the primary costimulatory molecule for naïve T cells, although CD4+ helper T cells are more dependent than are CD8+ killer T cells on CD28 costimulation. CD28 binds the CD80 (B7-1) and CD86 (B7-2) ligands that are expressed on antigen presenting cells (APCs). CD28 costimulation increases T cell responses in naïve cells by increasing cytokine (mainly IL-2) production, which results from an increase in both cytokine gene transcription and mRNA stabilization.
CD28 signaling involves the activation of the small Rho family GTPases Rac and CDC42, which activate p21-activated kinase. This may link them to the mitogen-activated protein kinase cascades and the subsequent induction of IL-2 synthesis. Rac and CDC42 are also important in CD28-mediated cytoskeletal rearrangements, through the action of the Wiscott-Aldrich syndrome protein (WASP).
CD28 costimulation increases the activity of nuclear transcription factors of the Rel/NFkB family, whose members bind the CD28-responsive element (CD28RE) present in several cytokine gene promoters.
CD28 triggering reduces the number of engaged TCRs necessary to induce cytokine production and cell proliferation. This threshold reduction for T-cell activation is attributed to CD28-induced recruitment of lipid rafts to the immunological synapse, which promotes recruitment of raft-associated kinase and adapter molecules.
▲ activation B : activation T : anergy : CD28 receptor : CD28RE : CDC42 : costimulatory molecules : first/second signals : helper T cell : IL-2 : MHC class II : negative regulators : plasma cells : Rac : regulatory mechanisms : Rel-NFkB : Rho GTPases : TCR engagement : TCR threshold reduction : WASP ▲
Tables Fc receptors Immune Cytokines Immunoglobulins
▲ Top ▲
tags [Immunology][costimulation][activation]

▼activation B : activation T : anergy : CD28 receptor : CD28RE : CDC42 : costimulatory molecules : first/second signals : helper T cell : IL-2 : MAPK cascade : MHC class II : negative regulators : Rac : regulatory mechanisms : Rel-NFkB : Rho GTPases : TCR engagement : TCR threshold reduction : transcription factors : WASP ▼
 Activation of B cells occurs when a BCR (antibody) encounters and ligates its cognate antigen. Naïve B cells each have one of millions of distinct surface antigen-specific receptors, yet have not encountered their specific, cognate antigen. With a life-span of only a few days, many B cells die without ever encountering their cognate antigen. In most cases, B-cell activation is dependent upon costimulation by an activated helper T cell that has itself been activated by the same antigen. (click images to enlarge)
Activation of B cells occurs when a BCR (antibody) encounters and ligates its cognate antigen. Naïve B cells each have one of millions of distinct surface antigen-specific receptors, yet have not encountered their specific, cognate antigen. With a life-span of only a few days, many B cells die without ever encountering their cognate antigen. In most cases, B-cell activation is dependent upon costimulation by an activated helper T cell that has itself been activated by the same antigen. (click images to enlarge)Unlike T cells, B cells are coated in immunoglobulin receptors and are able to recognize intact antigen, which they engulf, digest, and subsequently present in complex with surface MHC class II molecules. The MHC-peptide complex binds CD4 + helper T cells (Th), inducing secretion of cytokines that stimulate B cell proliferation and their differentiation into plasma cells, which secrete specific antibodies that bind with the cognate antigen. These antigen-antibody complexes are subsequently cleared by liver and spleen cells and the classical complement cascade.
 Activation of T cells requires (1) TCR engagement, which ensures antigen specificity and MHC restriction of the response. However, synergistic signaling by (2) costimulatory molecules is also necessary to sustain and integrate TCR signaling to stimulate optimal T cell proliferation and differentiation.
Activation of T cells requires (1) TCR engagement, which ensures antigen specificity and MHC restriction of the response. However, synergistic signaling by (2) costimulatory molecules is also necessary to sustain and integrate TCR signaling to stimulate optimal T cell proliferation and differentiation.Delivery of first signal (TCR engagement) in the absence of costimulation by a second signal(s) results in apoptosis or anergy. Anergic T cells neither produce IL-2 nor proliferate upon restimulation. This requirement of naïve T cell activation for delivery of both antigen-specific and costimulatory signals implies that only professional antigen presenting cells can initiate T cell responses.
Activation-regulatory mechanisms:
● increasing TCR avidity (adhesion molecules)
● enhancing recruitment of tyrosine kinases to the TCR complex coreceptors (CD4 and CD8)
● costimulation involving reciprocal and sequential signals between cells
Negative regulators of costimulation include receptors that bind B7 family members:
● CTLA-4
● PD-1
Molecules involved in costimulation include:
1. Disulfide-linked homodimers that bind to distinct members of the B7 family of surface proteins
---● CD28 ↓
---● ICOS (inducible costimulator) molecules
2. Members of the TNF receptor (TNFR) family
---● CD40, the major B cell costimulatory molecule
---● CD30
---● CD27
---● OX-40
---● 4-1BB
The CD28 receptor is involved in the best characterized costimulatory pathway. CD28 is the primary costimulatory molecule for naïve T cells, although CD4+ helper T cells are more dependent than are CD8+ killer T cells on CD28 costimulation. CD28 binds the CD80 (B7-1) and CD86 (B7-2) ligands that are expressed on antigen presenting cells (APCs). CD28 costimulation increases T cell responses in naïve cells by increasing cytokine (mainly IL-2) production, which results from an increase in both cytokine gene transcription and mRNA stabilization.
CD28 signaling involves the activation of the small Rho family GTPases Rac and CDC42, which activate p21-activated kinase. This may link them to the mitogen-activated protein kinase cascades and the subsequent induction of IL-2 synthesis. Rac and CDC42 are also important in CD28-mediated cytoskeletal rearrangements, through the action of the Wiscott-Aldrich syndrome protein (WASP).
CD28 costimulation increases the activity of nuclear transcription factors of the Rel/NFkB family, whose members bind the CD28-responsive element (CD28RE) present in several cytokine gene promoters.
CD28 triggering reduces the number of engaged TCRs necessary to induce cytokine production and cell proliferation. This threshold reduction for T-cell activation is attributed to CD28-induced recruitment of lipid rafts to the immunological synapse, which promotes recruitment of raft-associated kinase and adapter molecules.
▲ activation B : activation T : anergy : CD28 receptor : CD28RE : CDC42 : costimulatory molecules : first/second signals : helper T cell : IL-2 : MHC class II : negative regulators : plasma cells : Rac : regulatory mechanisms : Rel-NFkB : Rho GTPases : TCR engagement : TCR threshold reduction : WASP ▲
Tables Fc receptors Immune Cytokines Immunoglobulins
▲ Top ▲
tags [Immunology][costimulation][activation]
Labels: activation, B cells, BCR, co-stimulation, cognate antigen, first signal, immunoglobulin receptors, MHC, T cells, TCR







































